Description
*Read carefully the package insert before use.
Basic Performance Data
With Hitachi 7180 Automated Analyzer
Reproducibility
(mg/dL)
| Sample 1 | Sample 2 | Sample 3 | |
|---|---|---|---|
| n | 20 | 20 | 20 |
| Mean | 193.2 | 101.5 | 248.5 |
| S.D. | 0.89 | 1.05 | 1.70 |
| C.V. | 0.46 | 1.04 | 0.68 |
| Max. | 195 | 103 | 252 |
| Min. | 191 | 100 | 245 |
| Range | 4 | 3 | 7 |
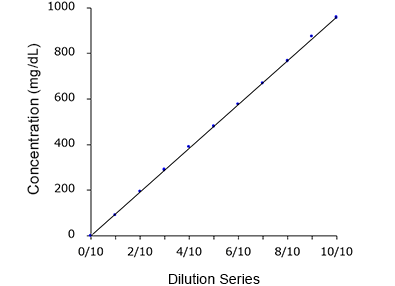
Linearity
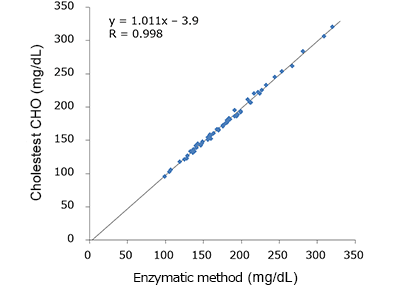
Correlation
Interference
Unconjugated bilirubin
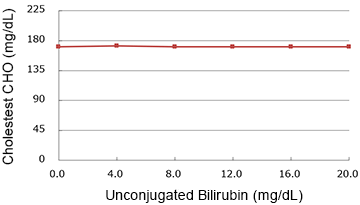
(mg/dL)
| Added Concentration (mg/dL) |
Measured Value | % |
|---|---|---|
| 0.0 | 171.0 | 100.0% |
| 4.0 | 172.0 | 100.6% |
| 8.0 | 171.0 | 100.0% |
| 12.0 | 171.0 | 100.0% |
| 16.0 | 171.0 | 100.0% |
| 20.0 | 171.0 | 100.0% |
Conjugated bilirubin
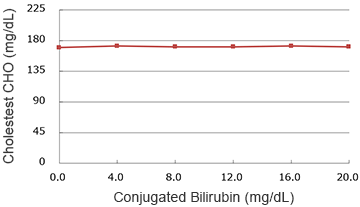
(mg/dL)
| Added Concentration (mg/dL) |
Measured Value | % |
|---|---|---|
| 0.0 | 170.0 | 100.0% |
| 4.0 | 172.0 | 101.2% |
| 8.0 | 171.0 | 100.6% |
| 12.0 | 171.0 | 100.6% |
| 16.0 | 172.0 | 101.2% |
| 20.0 | 171.0 | 100.6% |
Chyle
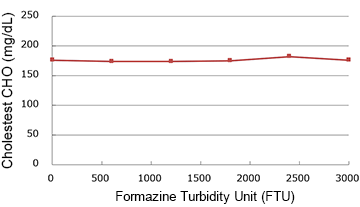
(mg/dL)
| Added Concentration (FTU) |
Measured Value | % |
|---|---|---|
| 0 | 176.0 | 100.0% |
| 600 | 174.0 | 98.9% |
| 1200 | 174.0 | 98.9% |
| 1800 | 175.0 | 99.4% |
| 2400 | 182.0 | 103.4% |
| 3000 | 176.0 | 100.0% |
Ascorbic acid
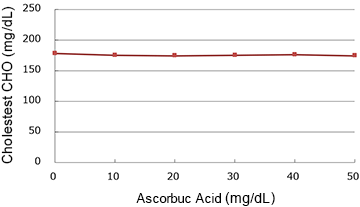
(mg/dL)
| Added Concentration (mg/dL) |
Measured Value | % |
|---|---|---|
| 0 | 178.0 | 100.0% |
| 10 | 175.0 | 98.3% |
| 20 | 174.0 | 97.8% |
| 30 | 175.0 | 98.3% |
| 40 | 176.0 | 98.9% |
| 50 | 174.0 | 97.8% |
Hemoglobin
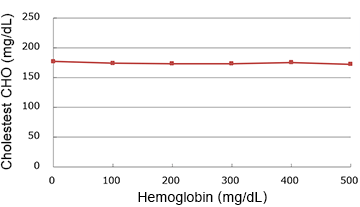
(mg/dL)
| Added Concentration (mg/dL) |
Measured Value | % |
|---|---|---|
| 0 | 177.0 | 100.0% |
| 100 | 174.0 | 98.3% |
| 200 | 173.0 | 97.7% |
| 300 | 173.0 | 97.7% |
| 400 | 175.0 | 98.9% |
| 500 | 172.0 | 97.2% |
Measurement Range(Hitachi 7150 Automated Analyzer)
5-1000mg/dL
Cholestest CHO