Description
*Read carefully the package insert before use.
Performance Data
Within-run reproducibility
| (%) | (INR) | |||
|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| 1 | 99.7 | 48.3 | 2.46 | 0.99 |
| 2 | 99.7 | 48.0 | 2.49 | 0.99 |
| 3 | 99.7 | 48.3 | 2.46 | 0.98 |
| 4 | 99.7 | 47.6 | 2.43 | 0.99 |
| 5 | 99.7 | 49.0 | 2.59 | 0.99 |
| 6 | 99.7 | 47.6 | 2.58 | 0.99 |
| 7 | 98.1 | 49.0 | 2.50 | 0.99 |
| 8 | 99.7 | 49.0 | 2.44 | 1.00 |
| 9 | 98.1 | 49.0 | 2.45 | 0.98 |
| 10 | 99.7 | 48.3 | 2.41 | 0.99 |
| Mean | 99.4 | 48.4 | 2.48 | 0.99 |
| S.D. | 0.67 | 0.57 | 0.06 | 0.01 |
| C.V. (%) | 0.67 | 1.2 | 2.45 | 0.57 |
| Max. | 99.7 | 49.0 | 2.59 | 1.00 |
| Min. | 98.1 | 47.6 | 2.41 | 0.98 |
| Range | 1.6 | 1.4 | 0.18 | 0.02 |
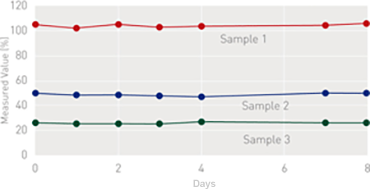
On-Board Stability (After Reconstitution)
[on Coapresta 2000]
Interference
| Concentration | Measured Value(%) | ||
|---|---|---|---|
| Base Plasma | Including Interfering Substance | ||
| Unconjugated Bilirubin |
20 mg/dL | 80.7 | 79.4 |
| Conjugated Bilirubin |
20 mg/dL | 79.4 | 80.0 |
| Hemoglobin | 500 mg/dL | 80.7 | 81.3 |
| Chyle | 3000 FTU | 80.0 | 80.7 |
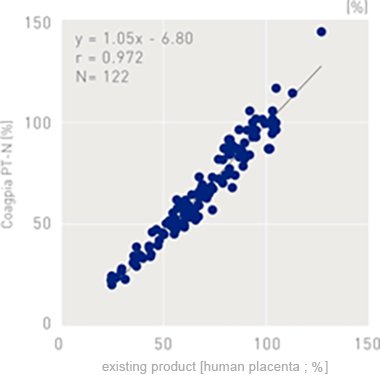
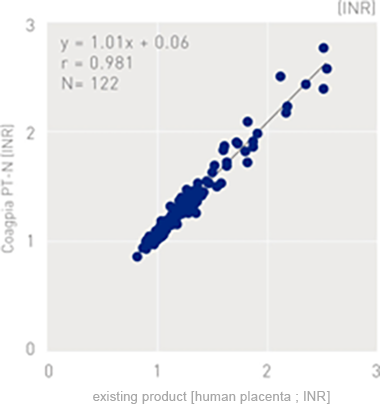
Correlation
Assay Procedure

Coagpia PT-N