Description
*Read carefully the package insert before use.
Performance Data
Within-run reproducibility
(µg/mL)
| Sample 1 | Sample 2 | |
|---|---|---|
| 1 | 3.5 | 9.5 |
| 2 | 3.6 | 9.5 |
| 3 | 3.5 | 9.4 |
| 4 | 3.4 | 9.3 |
| 5 | 3.5 | 9.5 |
| 6 | 3.5 | 9.3 |
| 7 | 3.5 | 9.4 |
| 8 | 3.6 | 9.5 |
| 9 | 3.5 | 9.3 |
| 10 | 3.5 | 9.4 |
| Mean | 3.5 | 9.4 |
| S.D. | 0.06 | 0.09 |
| C.V. (%) | 1.6 | 1.0 |
| Max. | 3.6 | 9.5 |
| Min. | 3.4 | 9.3 |
| Range | 0.2 | 0.2 |
On-Board Stability (After Reconstitution)
[on Coapresta 2000]
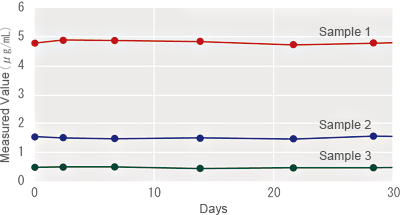
Interference
| Concentration | Measured Value(µg/mL) | ||
|---|---|---|---|
| Base Plasma | Including Interfering Substance | ||
| Unconjugated Bilirubin | 20mg/dL | 2.70 | 2.80 |
| Conjugated Bilirubin | 20mg/dL | 2.80 | 2.80 |
| Hemoglobin | 500mg/dL | 2.12 | 2.14 |
| Chyle | 2500 FTU | 2.17 | 2.04 |
Linearity
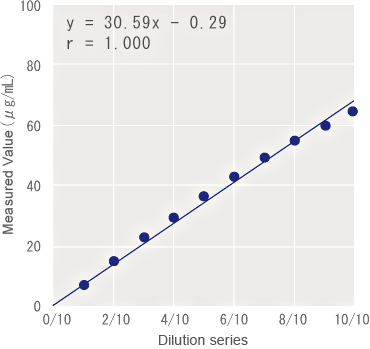
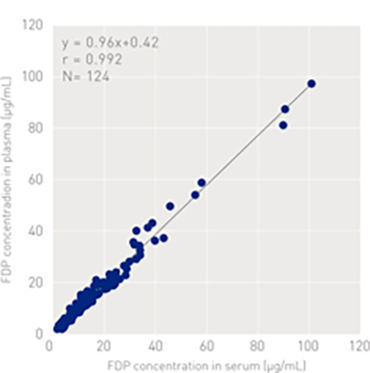
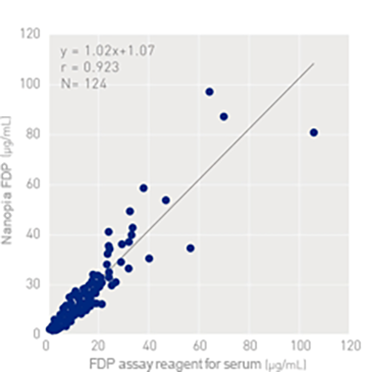
Correlation
Assay Procedure on Corepresta 2000
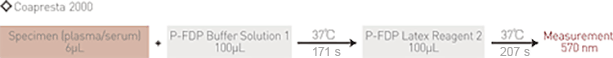
Nanopia P-FDP